Your Cell Therapy.
Automated Your Way.
Tools and Technologies for Every Workflow
CellBri’s solutions are engineered for speed, safety, and scalability, from benchtop prep to full-suite processing. This scalability empowers you to adapt and grow your operations as needed, putting you in control of your cell therapy development workflows.
The CellFAB One Series

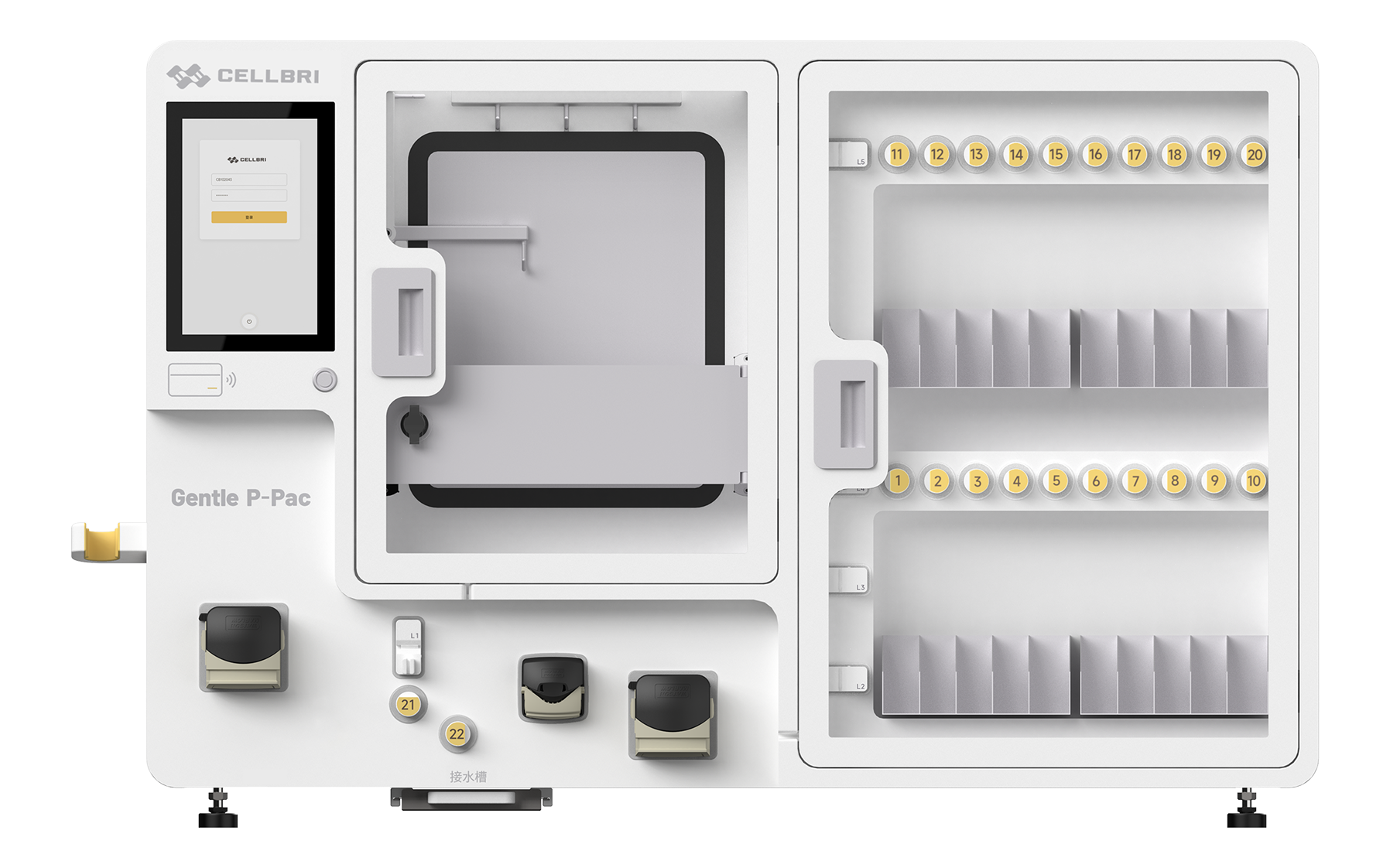
The Gentle Series